LifeScience Ledger(LSL) is a cloud-based platform that provides security, traceability and digital trust for the collection and reporting of clinical and healthcare data. LSL provides radical improvement in the elimination of manual validation steps through the application of digital trust.

Together, advancing compliant AI solutions for regulated industries.
We are excited to share that Claris AI, a trusted innovator in compliant AI solutions, has officially acquired BioVeras, a recognized leader in data-driven compliance and analytics. This partnership brings together two forward-thinking organizations with a shared mission to deliver tailored, cutting-edge solutions for regulated industries—such as life sciences, finance, and manufacturing—by combining BioVeras' regulatory expertise with Claris AI's advanced technology. Together, we are poised to help our clients navigate compliance with confidence and drive meaningful innovation.
This partnership brings new opportunities to enhance services and solutions for BioVeras customers and partners:
Expanded compliance and AI-driven solutions tailored to your needs.
Access to a broader range of tools and services designed for regulated industries.
Continued focus on integrity, innovation, and personalized support.
For more details, explore the links below:
BioVeras will transform the life sciences industry by enabling trusted, immutable data to become the gold standard for collaboration within the business and regulatory partners.
FIND OUT MORE
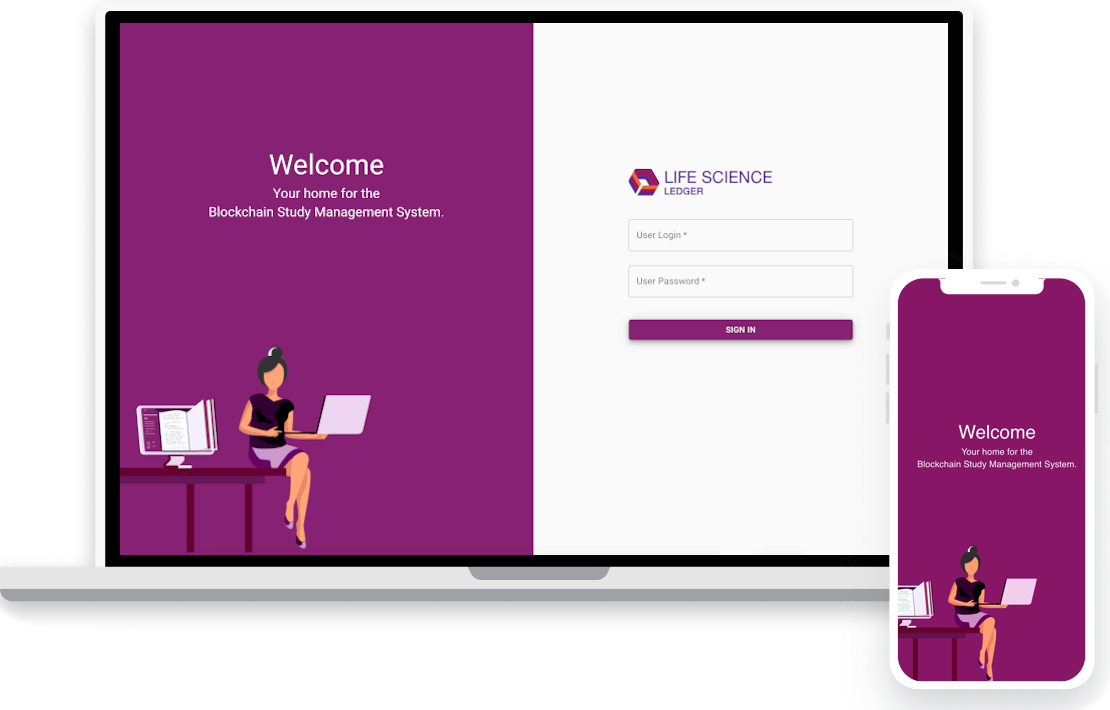
A clinical platform for CROs, Pharma, Biotech, and Med Device companies to manage many key aspects of the clinical trial process using Blockchain like technology
FIND OUT MORE
Our technology enables and facilitates multiple tasks in a traceable, validated environment that greatly improves the workflow and compliance of data collection and file creation - providing true TRACEABILITY and TRUST.

Secure, auditable data file transfer and compliance tool.

A Cloud-based, zero-footprint image collection, de-identification, transfer, file storage and retrieval solution.

An automated protocol “Exclusion, Inclusion” search tool to facilitate the identification and qualification of study participants.